| Structure | Name/CAS No. | Articles |
|---|---|---|
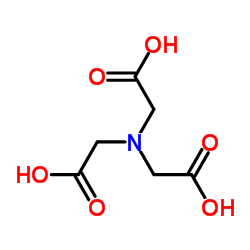 |
nitrilotriacetic acid
CAS:139-13-9 |
|
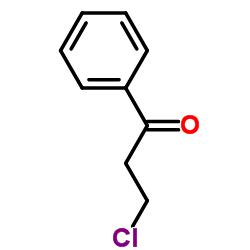 |
3-Chloropropiophenone
CAS:936-59-4 |
|
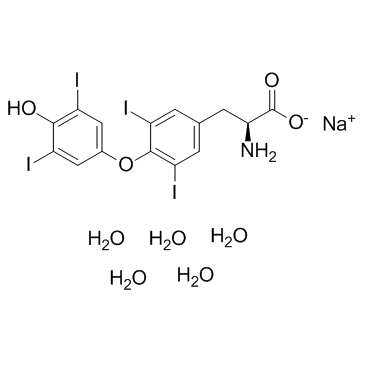 |
Sodium levothyroxine pentahydrate
CAS:6106-07-6 |
|
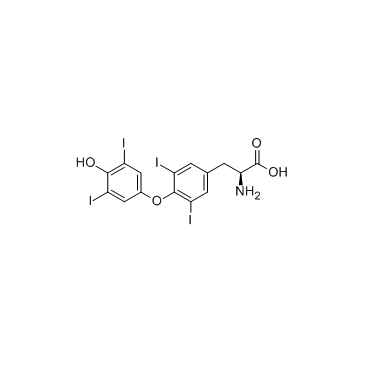 |
L-thyroxine
CAS:51-48-9 |