Organic Letters
1999-07-29
13C and 2H kinetic isotope effects and the mechanism of bromination of 1-pentene under synthetic conditions.
S R Merrigan, D A Singleton
Index: Org. Lett. 1(2) , 327-9, (1999)
Full Text: HTML
Abstract
[formula: see text] The 13C and 2H kinetic isotope effects for the bromination of 1-pentene with Br2 in CCl4 were determined and interpreted with the aid of calculationally predicted isotope effects. The isotope effects observed are consistent with rate-limiting bromonium ion formation and do not fit with either rate-limiting production of a pi complex or reaction of a reversibly formed bromonium ion. This rules out some of the mechanistic complexities suggested for other brominations, though the identity of the brominating reagent(s) under these synthetic conditions remains uncertain.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
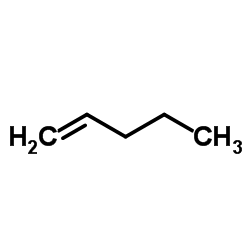 |
Pent-1-ene
CAS:109-67-1 |
C5H10 |
Related Articles:
More...
|
QSPR modeling of octanol/water partition coefficient for vit...
2008-04-01 [Eur. J. Med. Chem. 43 , 714-40, (2008)] |
|
OH-initiated photooxidations of 1-pentene and 2-methyl-2-pro...
2014-12-01 [ChemPhysChem 15(17) , 3848-54, (2014)] |
|
Use of olefin cross-metathesis to release azide-containing s...
2003-11-27 [Org. Lett. 5 , 4541, (2003)] |
|
TS-1 zeolite microengineered reactors for 1-pentene epoxidat...
2002-04-21 [Chem. Commun. (Camb.) (8) , 878-9, (2002)] |
|
Comparative safety analysis of surgical smoke from transuret...
2013-09-01 [Urology 82(3) , 744.e9-14, (2013)] |