| Structure | Name/CAS No. | Articles |
|---|---|---|
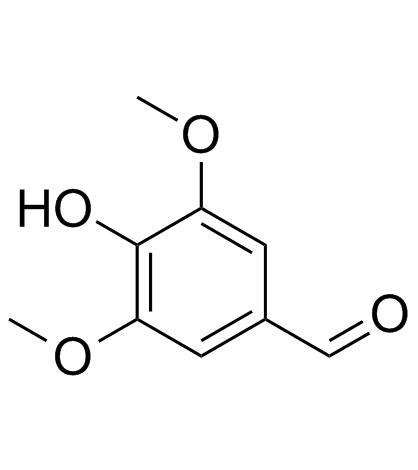 |
Syringaldehyde
CAS:134-96-3 |
|
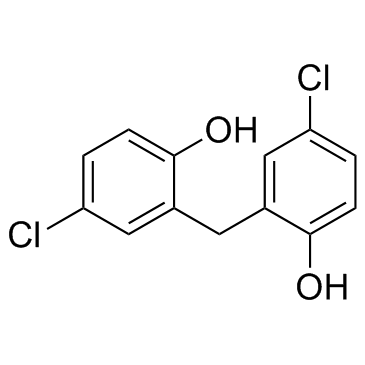 |
Dichlorophen
CAS:97-23-4 |
|
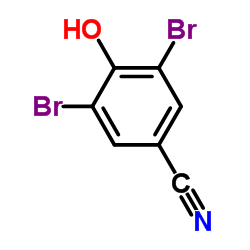 |
Bromoxynil
CAS:1689-84-5 |