[Nucleoside-5'-triphosphate hydrolysis in the liver and kidney of rats with chronic alloxan diabetes].
I M Rusina, A F Makarchikov, E A Makar, V L Kubyshin
Index: Biomed. Khim. 52 , 364-369, (2006)
Full Text: HTML
Abstract
Activity and some properties of a soluble enzyme hydrolyzing nucleoside-5'-triphosphates were studied in the liver and kidney of normal and diabetic rats. The enzyme activity was shown to be reduced by 34% (p < 0.01) in the liver extracts of diabetic animals, while no difference was observed in the kidney. When ITP was used as substrate, the apparent Michaelis constant of the enzyme was significantly lower in the liver of controls as compared to experimental rats (32.3 +/- 1.3 microM and 54.3 +/- 1.0 microM, respectively, p < 0.01). The KM values of the enzyme in the kidney were not distinguishable in both groups. NTPase exhibits maximal activity at pH 7.0 and has a broad substrate specificity with respect to different nucleoside-5'-tri- and diphosphates. Molecular mass of the enzyme was estimated by gel filtration to be 63.7 +/- 0.9 kD.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
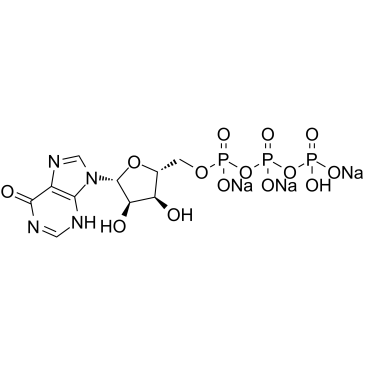 |
Inosine 5'-triphosphate (sodium salt)
CAS:35908-31-7 |
C10H12N4Na3O14P3 |
|
Mapping interactions between the Ca2+-ATPase and its substra...
2003-03-21 [J. Biol. Chem. 278 , 10112-10118, (2003)] |
|
Identification of an ITPase/XTPase in Escherichia coli by st...
2005-10-01 [Structure 13 , 1511-1520, (2005)] |
|
Trinitrophenyl-ATP and -ADP bind to a single nucleotide site...
1988-04-25 [J. Biol. Chem. 263 , 5569-5573, (1988)] |
|
Distinct interactions of G(salpha-long), G(salpha-short), an...
2002-08-15 [Biochem. Pharmacol. 64 , 583-593, (2002)] |
|
Characterization of an ecto-ATPase activity in Cryptococcus ...
2005-07-01 [FEMS Yeast Res. 5 , 899-907, (2005)] |