Identification and validation of an anthracycline/cyclophosphamide-based chemotherapy response assay in breast cancer.
Jude M Mulligan, Laura A Hill, Steve Deharo, Gareth Irwin, David Boyle, Katherine E Keating, Olaide Y Raji, Fionnuala A McDyer, Eamonn O'Brien, Max Bylesjo, Jennifer E Quinn, Noralane M Lindor, Paul B Mullan, Colin R James, Steven M Walker, Peter Kerr, Jacqueline James, Timothy S Davison, Vitali Proutski, Manuel Salto-Tellez, Patrick G Johnston, Fergus J Couch, D Paul Harkin, Richard D Kennedy
Index: J. Natl. Cancer Inst. 106(1) , djt335, (2014)
Full Text: HTML
Abstract
There is no method routinely used to predict response to anthracycline and cyclophosphamide-based chemotherapy in the clinic; therefore patients often receive treatment for breast cancer with no benefit. Loss of the Fanconi anemia/BRCA (FA/BRCA) DNA damage response (DDR) pathway occurs in approximately 25% of breast cancer patients through several mechanisms and results in sensitization to DNA-damaging agents. The aim of this study was to develop an assay to detect DDR-deficient tumors associated with loss of the FA/BRCA pathway, for the purpose of treatment selection.DNA microarray data from 21 FA patients and 11 control subjects were analyzed to identify genetic processes associated with a deficiency in DDR. Unsupervised hierarchical clustering was then performed using 60 BRCA1/2 mutant and 47 sporadic tumor samples, and a molecular subgroup was identified that was defined by the molecular processes represented within FA patients. A 44-gene microarray-based assay (the DDR deficiency assay) was developed to prospectively identify this subgroup from formalin-fixed, paraffin-embedded samples. All statistical tests were two-sided.In a publicly available independent cohort of 203 patients, the assay predicted complete pathologic response vs residual disease after neoadjuvant DNA-damaging chemotherapy (5-fluorouracil, anthracycline, and cyclophosphamide) with an odds ratio of 3.96 (95% confidence interval [Cl] =1.67 to 9.41; P = .002). In a new independent cohort of 191 breast cancer patients treated with adjuvant 5-fluorouracil, epirubicin, and cyclophosphamide, a positive assay result predicted 5-year relapse-free survival with a hazard ratio of 0.37 (95% Cl = 0.15 to 0.88; P = .03) compared with the assay negative population.A formalin-fixed, paraffin-embedded tissue-based assay has been developed and independently validated as a predictor of response and prognosis after anthracycline/cyclophosphamide-based chemotherapy in the neoadjuvant and adjuvant settings. These findings warrant further validation in a prospective clinical study.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
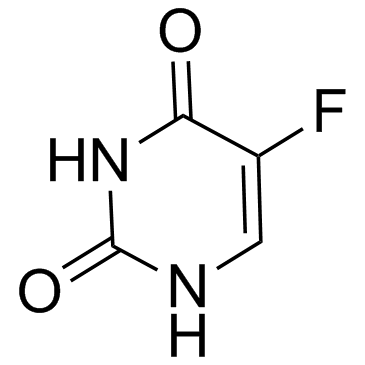 |
Fluorouracil
CAS:51-21-8 |
C4H3FN2O2 | |
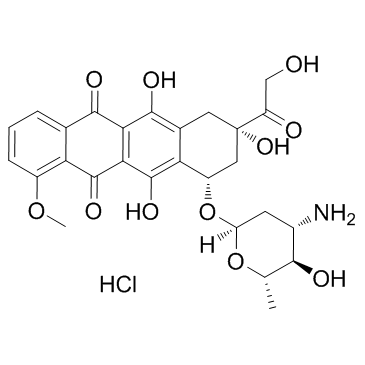 |
Epirubicin hydrochloride
CAS:56390-09-1 |
C27H30ClNO11 |
|
TAp73 promotes cell survival upon genotoxic stress by inhibi...
2014-09-30 [Oncotarget 5(18) , 8107-22, (2014)] |
|
cIEF for rapid pKa determination of small molecules: a proof...
2014-10-15 [Eur. J. Pharm. Sci. 63 , 14-21, (2014)] |
|
Establishment and characterization of cell lines from chromo...
2015-01-07 [World J. Gastroenterol. 21(1) , 164-76, (2015)] |
|
Targeted Delivery of 5-fluorouracil with Monoclonal Antibody...
[Iran. J. Pharm. Res. 14(2) , 395-405, (2015)] |
|
Atonal homolog 1 protein stabilized by tumor necrosis factor...
2015-08-01 [Cancer Sci. 106 , 1000-7, (2015)] |