18F-labeled insulin: a prosthetic group methodology for incorporation of a positron emitter into peptides and proteins.
Y Shai, K L Kirk, M A Channing, B B Dunn, M A Lesniak, R C Eastman, R D Finn, J Roth, K A Jacobson
Index: Biochemistry 28 , 4801, (1989)
Full Text: HTML
Abstract
In the present study we synthesize 18F-labeled insulin of high specific radioactivity. A new prosthetic group methodology, in which [18F]fluoride displaces a bromide group of 4-(bromomethyl)-benzoylamine intermediates, was used. The 4-(fluoromethyl)benzoyl product was chemically stable. 18F-Labeled insulin retains the essential biological properties of native insulin, as measured in vitro by binding to insulin receptors on human cells and stimulation of glucose metabolism in rat adipocytes. The overall process can be carried out speedily to yield a product of sufficient purity to permit in vivo studies. The method appears to be applicable to a wide variety of peptides.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
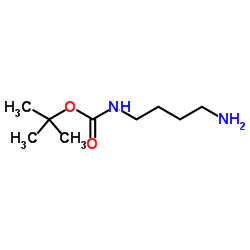 |
NH2-C4-NH-Boc
CAS:68076-36-8 |
C9H20N2O2 |
|
Peptide splicing in a double-sequence analogue of trypsin in...
2015-05-01 [Biopolymers 104 , 206-12, (2015)] |
|
Synthesis, tubulin binding, antineoplastic evaluation, and s...
1989-02-01 [J. Med. Chem. 32 , 409, (1989)] |
|
Synthesis and biological evaluation of new citrate-based sid...
2002-05-09 [J. Med. Chem. 45 , 2056, (2002)] |
|
Rationally designed peptoids modulate aggregation of amyloid...
2014-07-16 [ACS Chem. Neurosci. 5(7) , 552-8, (2014)] |
|
Synthesis of Amphiphilic Block Copolypept(o)ides by Bifuncti...
2015-12-01 [Macromol. Rapid Commun. 36 , 2083-91, (2016)] |