N(G)-Acyl-argininamides as NPY Y(1) receptor antagonists: Influence of structurally diverse acyl substituents on stability and affinity.
Stefan Weiss, Max Keller, Günther Bernhardt, Armin Buschauer, Burkhard König
Index: Bioorg. Med. Chem. 18(17) , 6292-304, (2010)
Full Text: HTML
Abstract
N(G)-Acylated argininamides, covering a broad range of lipophilicity (calculated logD values: -1.8-12.5), were synthesized and investigated for NPY Y(1) receptor (Y(1)R) antagonism, Y(1)R affinity and stability in buffer (N(G)-deacylation, yielding BIBP 3226). Broad structural variation of substituents was tolerated. The K(i) (binding) and K(b) values (Y(1)R antagonism) varied from low nM to one-digit muM. Most of the compounds proved to be sufficiently stable at pH 7.4 over 90min to determine reliable pharmacological data in vitro. Exceptionally high instability was detected when a succinyl moiety was attached to the guanidine, probably, due to an intramolecular cleavage mechanism.Copyright 2010 Elsevier Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
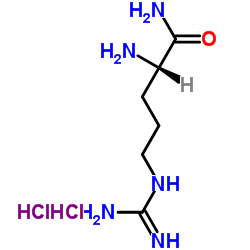 |
H-Arg-NH2.2HCl
CAS:14975-30-5 |
C6H17Cl2N5O |
|
Immobilization of trypsin in organic and aqueous media for e...
2015-01-01 [BMC Biotechnol. 15 , 77, (2015)] |
|
Energetic basis of molecular recognition in a DNA aptamer.
2007-03-01 [Biophys. Chem. 126(1-3) , 165-75, (2007)] |
|
Structural features of the L-argininamide-binding DNA aptame...
2006-10-15 [Anal. Chem. 78(20) , 7259-66, (2006)] |
|
Red-fluorescent argininamide-type NPY Y1 receptor antagonist...
2011-05-01 [Bioorg. Med. Chem. 19(9) , 2859-78, (2011)] |
|
Biomolecular sensor based on fluorescence-labeled aptamer.
2006-08-15 [Bioorg. Med. Chem. Lett. 16(16) , 4381-4, (2006)] |