Identification of the catalytic histidine residue participating in the charge-relay system of carboxypeptidase Y.
G Jung, H Ueno, R Hayashi, T H Liao
Index: Protein Sci. 4(11) , 2433-5, (1995)
Full Text: HTML
Abstract
The essential histidine residue of carboxypeptidase Y (CPY) was modified by a site-specific reagent, a chloromethylketone derivative of benzyloxycarbonyl-L-phenylalanine. The single modified histidine residue was converted to N tau-carboxy-methyl histidine (cmHis) upon performic acid oxidation. A peptide containing cmHis was isolated from the tryptic-thermolytic digest. Based on the amino acid composition and sequence analysis, the peptide is shown to be Val-Phe-Asp-Gly-Gly-cmHis-MetO2-Val-Pro, which was derived from CPY cleaved by trypsin at Arg 391 and thermolysin at Phe 401, and thus His 397 was modified. This histidine residue has been implicated previously by X-ray analysis to participate in the charge-relay system of CPY.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
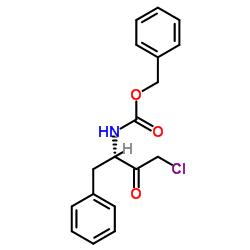 |
zpck
CAS:26049-94-5 |
C18H18ClNO3 |
|
High-content positional biosensor screening assay for compou...
2014-09-01 [Assay Drug Dev. Technol. 12(7) , 395-418, (2014)] |
|
Inhibition of carboxypeptidase Y by chloromethyl ketone deri...
1974-12-01 [J. Biochem. (Tokyo) 76 , 1355, (1974)] |
|
A chemical-genetic interaction map of small molecules using ...
2015-12-01 [Mol. Syst. Biol. 11 , 846, (2015)] |
|
Inactivation of chymotrypsin and human skin chymase: kinetic...
1988-04-14 [Biochim. Biophys. Acta 953(3) , 269-79, (1988)] |
|
Effect of protease inhibitors and substrates on 3,5,3'-triio...
1992-09-01 [Endocr. Regul. 26 , 127-131, (1992)] |