Spectroscopic and theoretical studies on the nucleophilic substitution of 2,3-dichloronaphthoquinone with para-substituted anilines in solid state via initial charge transfer complexation.
Angupillai Satheshkumar, Kuppanagounder P Elango
Index: Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 98 , 378-83, (2012)
Full Text: HTML
Abstract
Various spectroscopy techniques (UV-Vis, DRS, FT-IR, (1)H NMR, LC-MS) and theoretical computations have been employed to investigate the mechanism of the nucleophilic substitution reaction of 2,3-dichloronaphthoquinone (DCNQ) with para-substituted anilines in solid state under base- and solvent-free conditions against traditional synthetic routes. The initial formations of electron donor acceptor (EDA) adduct between DCNQ and aniline was found to be the driving force for the substitution reaction to occur in solid phase.Copyright © 2012 Elsevier B.V. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
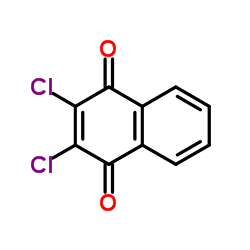 |
2,3-Dichlor-1,4-naphthochinone
CAS:117-80-6 |
C10H4Cl2O2 |
|
Indoleamine 2,3-dioxygenase is the anticancer target for a n...
2008-03-27 [J. Med. Chem. 51 , 1706-18, (2008)] |
|
The 1,4-naphthoquinone scaffold in the design of cysteine pr...
2007-08-01 [Bioorg. Med. Chem. 15 , 5340-50, (2007)] |
|
Modulation of hepatic cytochrome P-450 and DT-diaphorase by ...
1988-08-01 [Bull. Environ. Contam. Toxicol. 41(2) , 164-71, (1988)] |
|
Study of a reaction between 2,3-dichloro-1,4-naphthoquinone ...
1993-01-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 60(7) , 1641-7, (2004)] |
|
Charge transfer interaction of 4-acetamidophenol (paracetamo...
2008-12-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 71(3) , 835-40, (2008)] |