Inhibitory effect of ammonium tetrathiotungstate on tyrosinase and its kinetic mechanism.
Kyung-Hee Park, Jae-Rin Lee, Hwa-Sun Hahn, Young-Hoon Kim, Chang-Dae Bae, Jun-Mo Yang, Sangtaek Oh, Yu-Jin Bae, Dong-Eun Kim, Myong-Joon Hahn
Index: Chem. Pharm. Bull. 54(9) , 1266-70, (2006)
Full Text: HTML
Abstract
Tyrosinase requires two copper ions at the active site, in order to oxidize phenols to catechols. In this study, the inhibitory effect of the copper-chelating compound, ammonium tetrathiotungstate (ATTT), on the tyrosinase activity was investigated. ATTT was determined to inactivate the activity of mushroom tyrosinase, in a dose-dependent manner. The kinetic substrate reaction revealed that ATTT functions as a kinetically competitive inhibitor in vitro, and that the enzyme-ATTT complex subsequently undergoes a reversible conformational change, resulting in the inactivation of tyrosinase. In human melanin-producing cells, ATTT evidenced a more profound tyrosinase-inhibitory effect than has been seen in the previously identified tyrosinase inhibitors, including kojic acid and hydroquinone. Our results may provide useful information for the development of whitening agent.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
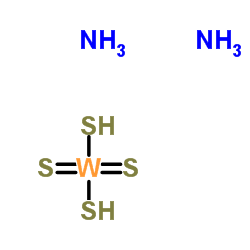 |
Disulfanyl(dithioxo)tungsten diammoniate
CAS:13862-78-7 |
H8N2S4W |
|
Heterogeneous WSx/WO₃ Thorn-Bush Nanofiber Electrodes for So...
2016-03-22 [ACS Nano 10 , 3257-66, (2016)] |
|
Behavior of [185W]thiotungstates injected into sheep and the...
1989-02-01 [J. Inorg. Biochem. 35(2) , 115-26, (1989)] |
|
Antitumor and antiinflammatory effects of tetrathiotungstate...
2007-05-01 [Transl. Res. 149(5) , 260-4, (2007)] |
|
Effects of tetrathiotungstate and dithiotungstate on copper ...
1982-04-01 [J. Inorg. Biochem. 16(2) , 121-34, (1982)] |
|
Lang, J-P. Tatsumi, K.
[Inorg. Chem. 37 , 6308, (1998)] |