The enzymology of cystathionine biosynthesis: strategies for the control of substrate and reaction specificity.
Susan M Aitken, Jack F Kirsch
Index: Arch. Biochem. Biophys. 433 , 166-175, (2005)
Full Text: HTML
Abstract
The ability of enzymes to catalyze specific reactions, while excluding others, is central to cellular metabolism. Control of reaction specificity is of particular importance for enzymes that employ catalytically versatile cofactors, of which pyridoxal 5'-phosphate is a prime example. Cystathionine gamma-synthase and cystathionine beta-synthase are the first enzymes in the transsulfuration and reverse transsulfuration pathways, respectively. Each of them occupies branch-point positions in amino acid metabolism and as such are subject to transcriptional and post-translational regulation. Both enzymes catalyze the pyridoxal 5'-phosphate-dependent formation of l-cystathionine; however, their substrate and reaction specificities are distinct. The mechanisms whereby these enzymes control the chemistry of the cofactor are the subject of this review.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
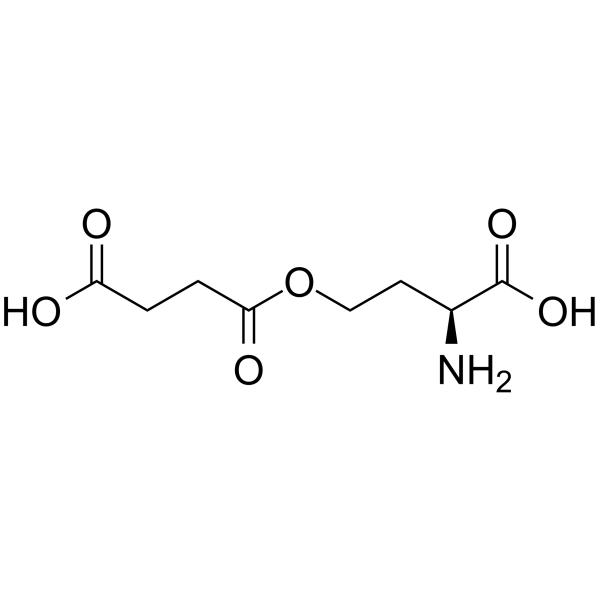 |
O-SUCCINYL-L-HOMOSERINE
CAS:1492-23-5 |
C8H13NO6 |
|
Cloning and characterization of two Lactobacillus casei gene...
2008-01-01 [Appl. Environ. Microbiol. 74 , 99-106, (2008)] |
|
Enzymatic characterization and inhibitor discovery of a new ...
2008-01-01 [J. Biochem. 143 , 59-68, (2008)] |
|
Pathways of assimilative sulfur metabolism in Pseudomonas pu...
1999-09-01 [J. Bacteriol. 181(18) , 5833-7, (1999)] |
|
Cloning and characterization of the CYS3 (CYI1) gene of Sacc...
1992-05-01 [J. Bacteriol. 174(10) , 3339-47, (1992)] |
|
A direct sulfhydrylation pathway is used for methionine bios...
1995-02-01 [Microbiology 141 ( Pt 2) , 431-9, (1995)] |