| Structure | Name/CAS No. | Articles |
|---|---|---|
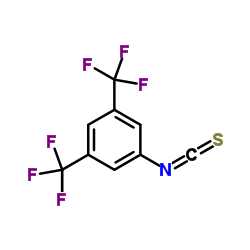 |
3,5-di(trifluoromethyl)phenyl isothiocyanate
CAS:23165-29-9 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
3,5-di(trifluoromethyl)phenyl isothiocyanate
CAS:23165-29-9 |