Carbon protonation of 2,4,6-triaminopyrimidines: synthesis, NMR studies, and theoretical calculations.
Balázs Németh, Csaba Wéber, Tamás Veszprémi, Tamás Gáti, Adám Demeter
Index: J. Org. Chem. 71(13) , 4910-8, (2006)
Full Text: HTML
Abstract
Several C5-substituted 2,4,6-triaminopyrimidine derivatives and their HBF4 salts were synthesized to study the carbon protonation of the pyrimidine ring. NMR investigations in DMSO-d6 prove experimentally that, in addition to the usual protonation at N1, the compounds can be protonated at C5 as well. We present several new stable cationic sigma-complexes in the pyrimidine series, where C5 protonation predominates over N1 protonation. Quantum chemical calculations using the B3LYP/cc-pVDZ method were utilized in the gas phase and also in DMSO solvent with the polarized continuum model (PCM) method to rationalize the observed protonation behavior. Results of the calculations accord with the experimental observations and prove that combined steric and electronic effects are responsible for the observed C5 protonation and for sigma-complex stability. We demonstrate that C5 protonation is a general feature of the 2,4,6-triaminopyrimidine system.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
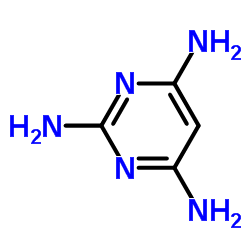 |
Pyrimidin-2,4,6-triamin
CAS:1004-38-2 |
C4H7N5 |
|
Determination of the driving force for the sodium pump (ENa)...
1987-01-01 [Gen. Pharmacol. 18(6) , 589-92, (1987)] |
|
Mechanism of ammonium transport by intestinal segments follo...
1992-08-01 [J. Urol. 148(2 Pt 1) , 453-7, (1992)] |
|
Factors affecting the potassium concentration at the mucosal...
1990-01-01 [Gut 31(1) , 64-9, (1990)] |
|
Competitive blocking of epithelial sodium channels by organi...
1983-01-01 [J. Membr. Biol. 76(3) , 235-51, (1983)] |
|
Synthesis and biological evaluation of novel pyrimidine deri...
2011-01-01 [Bioorg. Med. Chem. Lett. 21(23) , 7210-5, (2011)] |