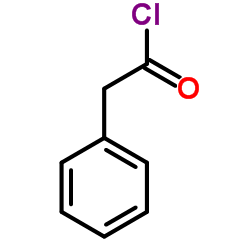
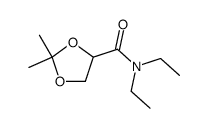
|
~% |
|
~% |
|
~% |
|
~68% |
|
~% |
|
~% |
|
~% |

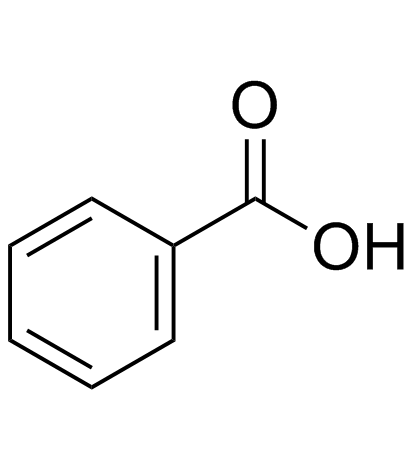


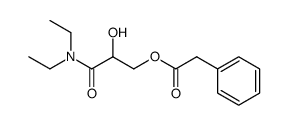
![[3-(diethylamino)-2,3-dioxopropyl] 2-phenylacetate Structure](https://image.chemsrc.com/caspic/266/497872-20-5.png)