| Structure | Name/CAS No. | Articles |
|---|---|---|
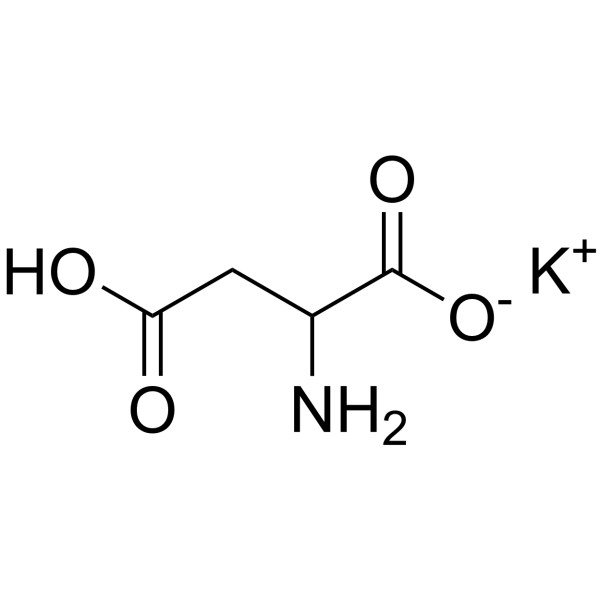 |
aspartic acid, potassium salt, l-
CAS:14434-35-6 |
|
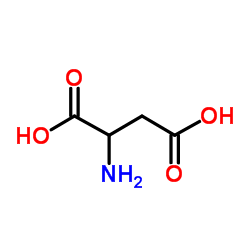 |
DL-Aspartic Acid
CAS:617-45-8 |
|
 |
DL-Alanine
CAS:302-72-7 |