| Structure | Name/CAS No. | Articles |
|---|---|---|
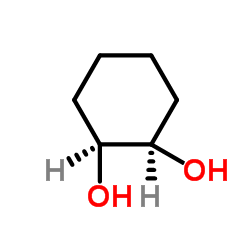 |
1,2-cis-cyclohexanediol
CAS:1792-81-0 |
|
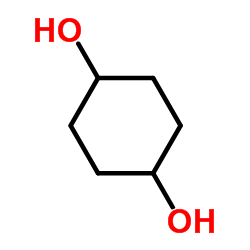 |
1,4-Cyclohexanediol
CAS:556-48-9 |
|
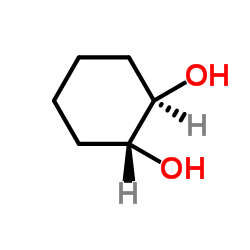 |
trans-Cyclohexane-1,2-diol
CAS:1460-57-7 |