Biochemistry and Molecular Biology International
1998-09-01
Various forms of rabbit plasma alpha-1-antiproteinase.
A Saito, H Sinohara
Index: Biochem. Mol. Biol. Int. 46(1) , 27-34, (1998)
Full Text: HTML
Abstract
Amino acid sequencing of the ficin-derived C-terminal fragments of alpha-1-antiproteinase (also called alpha-1-antitrypsin or alpha-1-proteinase inhibitor) prepared from rabbit plasma revealed the presence of the E isoform, which had been confirmed in the cDNA library in addition to the F and S-1 forms. The S-2 form was identified in inflamed rabbit plasma. The ficin digest of human plasma alpha-1-antitrypsin resulted in a major fragment of the M type. Multiple forms of alpha-1-antiproteinase in the rabbit plasma implicate the unknown functions other than the inhibition of neutrophil elastase.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
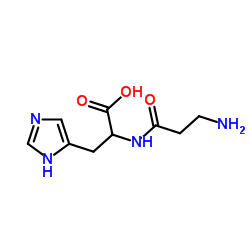 |
FICIN
CAS:9001-33-6 |
C9H14N4O3 |
Related Articles:
More...
|
Characterization of acid-induced molten globule like state o...
2009-10-01 [Int. J. Biol. Macromol. 45(3) , 248-54, (2009)] |
|
Variation in aspects of cysteine proteinase catalytic mechan...
2001-07-15 [Biochem. J. 357(Pt 2) , 343-52, (2001)] |
|
Production and characterization of two variants of human cys...
1998-04-15 [Arch. Biochem. Biophys. 352(2) , 199-206, (1998)] |
|
Persistence of cefotetan on red blood cells.
2004-06-01 [Transfusion 44(6) , 849-52, (2004)] |
|
A major target for warm immunoglobulin G autoantibodies: the...
2013-09-01 [Transfusion 53(9) , 1948-55, (2013)] |