(Photo)physical Properties of New Molecular Glasses End-Capped with Thiophene Rings Composed of Diimide and Imine Units.
Marzena Grucela-Zajac, Katarzyna Bijak, Slawomir Kula, Michal Filapek, Malgorzata Wiacek, Henryk Janeczek, Lukasz Skorka, Jacek Gasiorowski, Kurt Hingerl, NiyaziSerdar Sariciftci, Natalia Nosidlak, Gabriela Lewinska, Jerzy Sanetra, Ewa Schab-Balcerzak
Index: J. Phys. Chem. C Nanomater. Interfaces 118(24) , 13070-13086, (2014)
Full Text: HTML
Abstract
New symmetrical arylene bisimide derivatives formed by using electron-donating-electron-accepting systems were synthesized. They consist of a phthalic diimide or naphthalenediimide core and imine linkages and are end-capped with thiophene, bithiophene, and (ethylenedioxy)thiophene units. Moreover, polymers were obtained from a new diamine, N,N'-bis(5-aminonaphthalenyl)naphthalene-1,4,5,8-dicarboximide and 2,5-thiophenedicarboxaldehyde or 2,2'-bithiophene-5,5'-dicarboxaldehyde. The prepared azomethine diimides exhibited glass-forming properties. The obtained compounds emitted blue light with the emission maximum at 470 nm. The value of the absorption coefficient was determined as a function of the photon energy using spectroscopic ellipsometry. All compounds are electrochemically active and undergo reversible electrochemical reduction and irreversible oxidation processes as was found in cyclic voltammetry and differential pulse voltammetry (DPV) studies. They exhibited a low electrochemically (DPV) calculated energy band gap (E g) from 1.14 to 1.70 eV. The highest occupied molecular orbital and lowest unoccupied molecular orbital levels and E g were additionally calculated theoretically by density functional theory at the B3LYP/6-31G(d,p) level. The photovoltaic properties of two model compounds as the active layer in organic solar cells in the configuration indium tin oxide/poly(3,4-(ethylenedioxy)thiophene):poly(styrenesulfonate)/active layer/Al under an illumination of 1.3 mW/cm(2) were studied. The device comprising poly(3-hexylthiophene) with the compound end-capped with bithiophene rings showed the highest value of V oc (above 1 V). The conversion efficiency of the fabricated solar cell was in the range of 0.69-0.90%.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
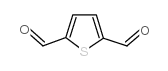 |
2,5-Thiophenedicarboxaldehyde
CAS:932-95-6 |
C6H4O2S |
|
Chiral Synthesis via Organoboranes. 46. An Efficient Prepara...
2001-01-01 [J. Org. Chem. 64(3) , 721-725, (1999)] |
|
Structure-in vitro activity relationships of pentamidine ana...
1998-10-01 [Antimicrob. Agents Chemother. 42(10) , 2495-502, (1998)] |
|
Tunable electroluminescence from silicon-containing poly (p-...
[Macromolecules 31(4) , 1114-23, (1998)] |
|
The Preparation of 2, 5-Thiophenedicarboxaldehyde. Sone T.
[Bull. Chem. Soc. Jpn. 37(8) , 1197-1200, (1964)] |