Oxidation of nitrotoluenes by toluene dioxygenase: evidence for a monooxygenase reaction.
J B Robertson, J C Spain, J D Haddock, D T Gibson
Index: Appl. Environ. Microbiol. 58(8) , 2643-8, (1992)
Full Text: HTML
Abstract
Pseudomonas putida F1 and Pseudomonas sp. strain JS150 initiate toluene degradation by incorporating molecular oxygen into the aromatic nucleus to form cis-1,2-dihydroxy-3-methylcyclohexa-3,5-diene. When toluene-grown cells were incubated with 2- and 3-nitrotoluene, the major products identified were 2- and 3-nitrobenzyl alcohol, respectively. The same cells oxidized 4-nitrotoluene to 2-methyl-5-nitrophenol and 3-methyl-6-nitrocatechol. Escherichia coli JM109(pDTG601), which contains the toluene dioxygenase genes from P. putida F1 under the control of the tac promoter, oxidized the isomeric nitrotoluenes to the same metabolites as those formed by P. putida F1 and Pseudomonas sp. strain JS150. These results extend the range of substrates known to be oxidized by this versatile enzyme and demonstrate for the first time that toluene dioxygenase can oxidize an aromatic methyl substituent.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
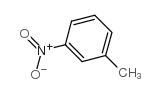 |
3-Nitrotoluene
CAS:99-08-1 |
C7H7NO2 |
|
Optimizations of packed sorbent and inlet temperature for la...
2014-08-22 [J. Chromatogr. A. 1356 , 221-9, (2014)] |
|
Hexafluoroisopropanol-modified cetyltrimethylammonium bromid...
2015-07-24 [J. Chromatogr. A. 1404 , 131-40, (2015)] |
|
Micellar electrokinetic chromatography of organic and peroxi...
2015-05-30 [Anal. Chim. Acta 876 , 91-7, (2015)] |
|
Comparative toxicities of o-, m-, and p-nitrotoluene in 13-w...
1994-04-01 [Fundam. Appl. Toxicol. 22(3) , 411-21, (1994)] |
|
Metabolism of nitrotoluenes by freshly isolated Fischer 344 ...
1984-01-01 [Drug Metab. Dispos. 12(1) , 45-50, (1984)] |