Journal of Chemical Information and Modeling
2012-02-27
Conformational analysis of 6α- and 6β-naltrexol and derivatives and relationship to opioid receptor affinity.
Jennifer A Bayron, Amy M Deveau, John M Stubbs
Index: J. Chem. Inf. Model. 52(2) , 391-5, (2012)
Full Text: HTML
Abstract
Naltrexol and its C₆ α and β desoxy, iodo, mesyl, tosyl, trifyl, dimethylcarbamyl, and diphenylcarbamyl derivatives were studied in their energy-minimized C ring chair-like and boat-like conformations using B3LYP/6-31G** and SM5.4/A to estimate aqueous solvation free energy. The results were compared to experimental opioid receptor binding affinities. The total energy difference between β conformers correlated well with MOR binding affinity, while the aqueous solvation free energy correlated well with the KOR binding affinity.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
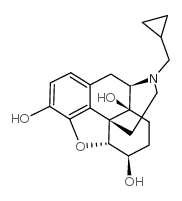 |
6β-Naltrexol
CAS:49625-89-0 |
C20H25NO4 |
Related Articles:
More...
|
In vivo evaluation of a transdermal codrug of 6-beta-naltrex...
2008-04-23 [Eur. J. Pharm. Sci. 33(4-5) , 371-9, (2008)] |
|
Comparison of naltrexone, 6alpha-naltrexol, and 6beta-naltre...
2008-01-01 [Psychopharmacology 195(4) , 479-86, (2008)] |
|
Comparison of the opioid receptor antagonist properties of n...
2008-03-31 [Eur. J. Pharmacol. 583(1) , 48-55, (2008)] |
|
An automated, highly sensitive LC-MS/MS assay for the quanti...
2008-10-15 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 874(1-2) , 33-41, (2008)] |
|
Determination of naltrexone and 6beta-naltrexol in human blo...
2010-02-01 [Anal. Bioanal. Chem 396(3) , 1249-57, (2010)] |