Study of 1,3,5-triazine-based catalytic amide-forming reactions: effect of solvents and basicity of reactants.
Munetaka Kunishima, Masanori Kitamura, Hiroyuki Tanaka, Ichiro Nakakura, Takahiro Moriya, Kazuhito Hioki
Index: Chem. Pharm. Bull. 61(8) , 882-6, (2013)
Full Text: HTML
Abstract
Effect of the basic property of reactants (tertiary amine catalysts, a substrate amine, and acid neutralizers) on catalytic dehydrocondensation between a carboxylic acid and an amine by using 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) was studied. The reaction yield was affected by the acid-base equilibrium among reactants. In dichloromethane, a representative aprotic solvent, a strongly basic catalyst gave amides in higher yields than weakly basic catalysts, regardless of the basicity of the acid neutralizer, which is called the proton capture agent (PCA). In contrast, in protic solvents, such as methanol or aqueous methanol, weakly basic catalysts gave amides in somewhat better yields than the strongly basic catalysts. In general, PCAs with weakly basic properties are favorable, because those with strongly basic properties tend to give byproducts arising from the reaction between CDMT and the substrate amine.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
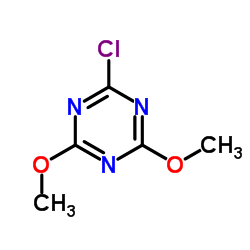 |
2-Chloro-4,6-dimethoxy-1,3,5-triazine
CAS:3140-73-6 |
C5H6ClN3O2 |
|
Towards the chiral metabolomics: Liquid chromatography-mass ...
2015-05-22 [Anal. Chim. Acta 875 , 73-82, (2015)] |
|
Study on 1,3,5-triazine chemistry in dehydrocondensation: ga...
2012-12-03 [Chemistry 18(49) , 15856-67, (2012)] |
|
Bis(4,6-dimethoxy-1,3,5-triazin-2-yl) ether as coupling reag...
2013-05-01 [Chem. Biodivers. 10(5) , 952-61, (2013)] |
|
Specific labeling of streptavidin for better understanding o...
2014-01-01 [Chem. Pharm. Bull. 62(11) , 1146-50, (2014)] |
|
Labeling study of avidin by modular method for affinity labe...
2010-12-01 [Bioorg. Med. Chem. Lett. 20 , 7050-3, (2010)] |