Journal of Organic Chemistry
2008-04-04
Zeolite NaY-promoted cyclization of farnesal: a short route to nanaimoal.
Constantinos Tsangarakis, Ioannis N Lykakis, Manolis Stratakis
Index: J. Org. Chem. 73(7) , 2905-8, (2008)
Full Text: HTML
Abstract
The sesquiterpene nanaimoal was synthesized in 21% overall yield and in a biomimetic manner. As a key step, the acid-catalyzed cyclization of farnesal under zeolite NaY confinement conditions was used. The intrazeolite cyclization of farnesal affords as major product a double-bond isomer of nanaimoal, via a novel diastereoselective tandem 1,5-diene cyclization/Prins-type reaction.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
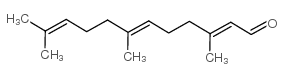 |
FARNESAL
CAS:19317-11-4 |
C15H24O |
Related Articles:
More...
|
Knockdown or inhibition of aldo-keto reductase 1B10 inhibits...
2014-12-28 [Cancer Lett. 355(2) , 273-80, (2014)] |
|
Formation of farnesal and 3-hydroxy-2,3-dihydrofarnesal from...
1993-11-15 [Biochem. Biophys. Res. Commun. 196(3) , 1401-5, (1993)] |
|
Chemical ecology of oribatid mites III. Chemical composition...
2003-01-01 [Exp. Appl. Acarol. 29(3-4) , 279-91, (2003)] |
|
Chromene-3-carboxamide derivatives discovered from virtual s...
2010-04-01 [Bioorg. Med. Chem. 18(7) , 2485-90, (2010)] |
|
Absolute configuration of chiral terpenes in marking pheromo...
2004-05-05 [Chirality 16(4) , 228-33, (2004)] |