Pre-Clinical Pharmacokinetic and Pharmacodynamic Characterization of Selected Chiral Flavonoids: Pinocembrin and Pinostrobin.
Casey L Sayre, Samaa Alrushaid, Stephanie E Martinez, Hope D Anderson, Neal M Davies
Index: J. Pharm. Pharm. Sci. 18 , 368-95, (2015)
Full Text: HTML
Abstract
Delineate the stereospecific pharmacokinetics and pharmacodynamics of the chiral flavonoids pinocembrin and pinostrobin.Characterize for the first time the stereoselective pharmacokinetics of two flavonoids, pinocembrin and pinostrobin and their bioactivity in several in vitro assays with relevant roles in heart disease, colon cancer, and diabetes etiology and pathophysiology.Chiral flavonoids were intravenously and orally administered to male Sprague-Dawley rats. Concentrations in serum and urine were characterized via stereospecific HPLC or LC/MS. Pure enantiomeric forms of each flavonoid were tested, where possible, to identify the stereospecific contribution to bioactivity in comparison to their racemates.Short half-lives (0.2-6 h) in serum were observed, while a better estimation of half-life (3-26 h) and other pharmacokinetic parameters were observed using urinary data. The flavonoids are predominantly excreted via non-renal routes (fe values of 0.3-4.6 %), and undergo rapid and extensive phase II metabolism. Chiral differences in the chemical structure of these compounds result in significant pharmacodynamic differences.The importance of understanding the stereospecific pharmacokinetics and pharmacodynamics of two chiral flavonoids were delineated.This article is open to POST-PUBLICATION REVIEW. Registered readers (see "For Readers") may comment by clicking on ABSTRACT on the issue's contents page.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
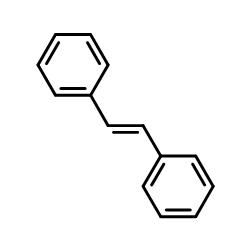 |
diphenylethylene
CAS:103-30-0 |
C14H12 |
|
Two-Photon Excitation of trans-Stilbene: Spectroscopy and Dy...
2015-07-23 [J. Phys. Chem. B 119 , 9335-44, (2015)] |
|
Freezing-in orientational disorder induces crossover from th...
2014-01-01 [Nat. Commun. 5 , 5642, (2014)] |
|
Kinetic studies of the reaction between pesticides and hydro...
2016-03-30 [J. Sci. Food Agric. 96 , 1580-4, (2016)] |
|
Harvest date affects aronia juice polyphenols, sugars, and a...
2015-11-15 [Food Chem. 187 , 189-96, (2015)] |
|
High-performance liquid chromatography separation of unsatur...
2015-08-01 [J. Sep. Sci. 38 , 2841-7, (2015)] |