| Structure | Name/CAS No. | Articles |
|---|---|---|
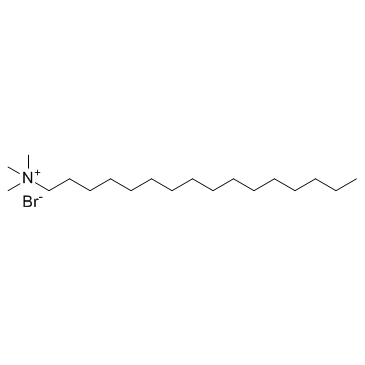 |
Hexadecyl trimethyl ammonium bromide
CAS:57-09-0 |
|
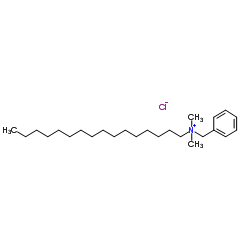 |
Cetalkonium chloride
CAS:122-18-9 |
|
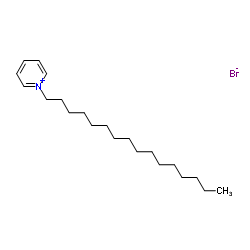 |
Cetylpyridinium Bromide
CAS:140-72-7 |