Three-dimensional collagen regulates collagen gene expression by a mechanism that requires serine/threonine kinases and is independent of mechanical contraction.
A Broberg, L Nissinen, M Potila, J Heino
Index: Biochem. Biophys. Res. Commun. 280(1) , 328-33, (2001)
Full Text: HTML
Abstract
Integrin alpha1beta1, one of the cellular collagen receptors, can participate in the regulation of collagen accumulation by acting as a negative feedback regulator. The molecular mechanism behind this phenomenon has been unknown. We have plated cells inside three-dimensional collagen and analyzed a set of chemical inhibitors for various signal transduction pathways. Only two wide-spectrum serine/threonine kinase inhibitors, H-7 and iso-H-7 could prevent the down-regulation of alpha1(I) collagen mRNA levels in cells exposed to three-dimensional collagen. In monolayer iso-H-7 slightly down-regulated collagen gene expression, indicating that inside collagen it affected integrin signaling rather than having a direct stimulatory effect on collagen mRNA levels. The effect of iso-H-7 was not dependent on its ability to inhibit protein kinases A, C, or G. H-7 and iso-H-7 could also inhibit collagen gel contraction, but this mechanism was independent of collagen gene regulation. Three-dimensional collagen could also up-regulate the mRNA levels of several matrix metalloproteinases (MMPs) but H-7 and iso-H-7 had no effect on the regulation of MMP genes. Our data indicate that three-dimensional collagenous matrix regulates distinct cellular signaling pathways and that collagen gene regulation is independent of the other effects of the matrix.Copyright 2001 Academic Press.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
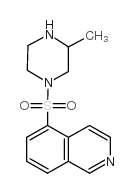 |
1-(5-ISOQUINOLINYLSULFONYL)-3-METHYL-PIPERAZINE
CAS:84477-73-6 |
C14H17N3O2S |
|
Production of interleukin (IL)-6 and IL-8 by a choriocarcino...
2000-05-01 [Placenta 21(4) , 354-60, (2000)] |
|
Epidermal growth factor-induced heterologous desensitization...
1999-01-01 [Endocrinology 140(1) , 29-36, (1999)] |
|
Alpha1-adrenergic regulation of peptidylglycine alpha-amidat...
1999-08-20 [Mol. Cell. Endocrinol. 154(1-2) , 89-100, (1999)] |
|
Protein kinase C inhibition blocks the early appearance of v...
1999-10-16 [Brain Res. 845(1) , 97-101, (1999)] |
|
The enhancing mechanism of capric acid (C10) from a supposit...
1997-04-01 [Biol. Pharm. Bull. 20(4) , 446-8, (1997)] |