Disposition of a 14C-labeled bioerodible polyorthoester and its hydrolysis products, 4-hydroxybutyrate and cis,trans-1,4-bis(hydroxymethyl)cyclohexane, in rats.
S L Sendelbeck, C L Girdis
Index: Drug Metab. Dispos. 13(3) , 291-5, (1985)
Full Text: HTML
Abstract
Disposition of the 14C-labeled bioerodible polymer poly(2,2-dioxy-cis,trans-1,4-cyclohexane dimethylene tetrahydrofuran) (ALZAMER C101ct) and its ultimate hydrolysis products, 4-hydroxybutyrate (4HB) and cis,trans-1,4-bis(hydroxymethyl)cyclohexane (CHDM), was assessed in vivo in rats 24 hr after sc administration of the 14C-labeled polymer or hydrolysis products. The hydrolysis products were rapidly metabolized and eliminated, whether administered directly or via in vivo erosion of the polymer. At 24 hr, fractional distribution of carbon-14 derived from 4HB was 65 to 75% in expired CO2, 5 to 10% in urine, 1 to 2% in feces, and 15 to 19% in tissues. For CHDM, distribution was 92 to 96% in urine, 2 to 3% in feces, and 0.3 to 3% in tissues. Hydrolysis products or metabolites were not sequestered in specific tissues. Pretreatment with unlabeled polymer for 28 days had little effect on the disposition of carbon-14. Minor differences in disposition between the polymer and its hydrolysis products are discussed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
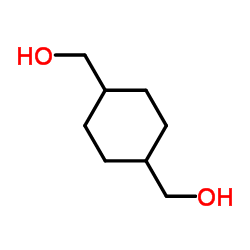 |
Cyclohexane-1,4-dimethanol
CAS:105-08-8 |
C8H16O2 |
|
Generation of a focused poly(amino ether) library: polymer-m...
2012-01-01 [Theranostics 2 , 1160-73, (2013)] |
|
A Processable Shape Memory Polymer System for Biomedical App...
2015-06-24 [Adv. Healthc. Mater. 4 , 1386-98, (2015)] |
|
Polyketal copolymers: a new acid-sensitive delivery vehicle ...
2008-06-01 [Bioconjug. Chem. 19(6) , 1164-9, (2008)] |
|
Single-step conversion of dimethyl terephthalate into cycloh...
2006-07-17 [Angew. Chem. Int. Ed. Engl. 45(29) , 4782-5, (2006)] |
|
Lack of androgenicity and estrogenicity of the three monomer...
2012-06-01 [Food Chem. Toxicol. 50(6) , 2196-205, (2012)] |