| Structure | Name/CAS No. | Articles |
|---|---|---|
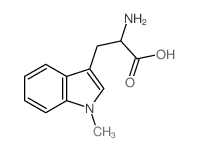 |
(Rac)-Indoximod
CAS:26988-72-7 |
|
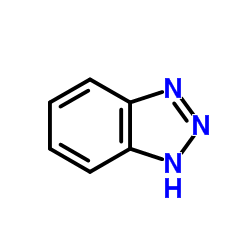 |
1H-Benzotriazole
CAS:95-14-7 |