Organic Letters
2008-03-20
SmI2-promoted Reformatsky-type coupling reactions in exceptionally hindered contexts.
Brian A Sparling, Ryan M Moslin, Timothy F Jamison
Index: Org. Lett. 10(6) , 1291-4, (2008)
Full Text: HTML
Abstract
Highly substituted, very hindered enones were synthesized using a two-step procedure that utilizes a diiodosamarium-promoted Reformatsky-type coupling and dehydration using Martin sulfurane. Both alpha-chloro- and alpha-bromoketones were coupled with a variety of carbonyl nucleophiles to form the intermediate beta-hydroxyketones, occurring with excellent diastereoselectivity, favoring the syn isomer (R1=Me). This technique complements other methods and enables the preparation of enones outside of the scope of current olefination methodology.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
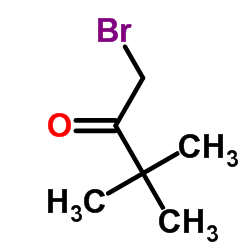 |
1-Brom-3,3-dimethylbutan-2-on
CAS:5469-26-1 |
C6H11BrO |
Related Articles:
More...
|
Synthesis and biological activity of an azido derivative of ...
1988-12-01 [Plant Physiol. 88(4) , 1425-9, (1988)] |
|
1-Bromopinacolone, an active site-directed covalent inhibito...
1982-12-10 [J. Biol. Chem. 257(23) , 14087-92, (1982)] |
|
Reactions of 1-bromo-2-[14C]pinacolone with acetylcholineste...
1989-08-31 [Biochim. Biophys. Acta 997(3) , 167-75, (1989)] |
|
Active-site peptides of acetylcholinesterase of Electrophoru...
1994-10-19 [Biochim. Biophys. Acta 1208(2) , 324-31, (1994)] |
|
Labeling of cysteine 231 in acetylcholinesterase from Torped...
1993-01-05 [J. Biol. Chem. 268(1) , 245-51, (1993)] |