| Structure | Name/CAS No. | Articles |
|---|---|---|
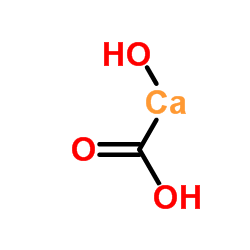 |
calcium carbonate
CAS:471-34-1 |
|
 |
Carbonic anhydrase
CAS:9001-03-0 |