Synthesis and biological activities of oxytocin and lysine vasopressin analogs containing glutamic acid gamma-hydrazide in position 4.
D Gazis, J Glass, I L Schwartz, G Stavropoulos, D Theodoropoulos
Index: Int. J. Pept. Protein Res. 34(5) , 353-7, (1989)
Full Text: HTML
Abstract
Solution methods, using N-hydroxysuccinimide esters, were used to synthesize [Glu(NHNH2)4] oxytocin and [Glu(NHNH2)4, Lys8] vasopressin. In these analogs of neurohypophyseal hormones, the side-chain carboxamide function of a glutamine residue is formally replaced by a hydrazide group at position 4. The hormone analogs were assayed for uterototonic activity, milk ejection activity, antidiuretic activity, and rat pressor activity. The specific biological activities of the oxytocin and vasopressin analogs were decreased compared to the respective parent hormones in all assay systems.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
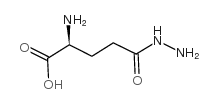 |
L-Glutamic acid,5-hydrazide
CAS:1820-73-1 |
C5H11N3O3 |
|
Modulation of lysine transport in cultured rat astrocytes an...
1987-01-01 [Membr. Biochem. 7(4) , 249-57, (1987)] |
|
Expression of the gltP gene of Escherichia coli in a glutama...
1995-11-01 [Mol. Microbiol. 18(4) , 641-7, (1995)] |
|
Cell differentiation of Proteus mirabilis is initiated by gl...
1993-04-01 [Mol. Microbiol. 8(1) , 53-60, (1993)] |
|
Alternative substrates for wild-type and L109A E. coli CTP s...
2004-11-01 [Eur. J. Biochem. 271(21) , 4204-12, (2004)] |
|
Stimulation by glutamine of the formation of N6-hydroxylysin...
1984-01-01 [J. Cell. Biochem. 24(4) , 395-403, (1984)] |