1,3-Dipolar cycloaddition-decarboxylation reactions of an azomethine ylide with isatoic anhydrides: formation of novel benzodiazepinones.
Asha M D'Souza, Nadia Spiccia, Jose Basutto, Pawel Jokisz, Leon S-M Wong, Adam G Meyer, Andrew B Holmes, Jonathan M White, John H Ryan
Index: Org. Lett. 13(3) , 486-9, (2011)
Full Text: HTML
Abstract
A nonstabilized azomethine ylide reacts with a wide range of substituted isatoic anhydrides to afford novel 1,3-benzodiazepin-5-one derivatives, which are generally isolated in high yield. The transformations involve 1,3-dipolar cycloaddition reactions of the ylide with the anhydrides to give transient, and in a representative case spectroscopically observable, oxazolidine intermediates that undergo ring-opening-decarboxylation-ring-closing reaction cascades to yield the 1,3-benzodiazepin-5-one products.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
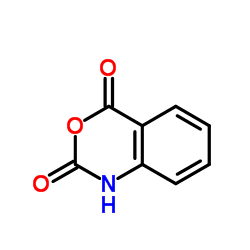 |
Isatoic anhydride
CAS:118-48-9 |
C8H5NO3 |
|
Hydrogen-bonding in 2-aminobenzoyl-alpha-chymotrypsin formed...
2002-01-04 [ChemBioChem. 3(1) , 68-75, (2002)] |
|
Palladium-catalyzed decarboxylative coupling of isatoic anhy...
2011-11-18 [Org. Lett. 13 , 6114-6117, (2011)] |
|
Suicide enzyme inactivators.
1983-01-01 [Basic Life Sci. 25 , 287-305, (1983)] |
|
Synthesis of 2,3-dihydroquinazolin-4(1H)-ones by three-compo...
2010-09-13 [J. Comb. Chem. 12(5) , 643-6, (2010)] |
|
Novel and efficient one-pot tandem synthesis of 2-styryl-sub...
2008-01-01 [J. Comb. Chem. 10(5) , 700-3, (2008)] |