Assay of the enantiomers of 1,2-propanediol, 1,3-butanediol, 1,3-pentanediol, and the corresponding hydroxyacids by gas chromatography-mass spectrometry.
L Powers, S T Ciraolo, K C Agarwal, A Kumar, C Bomont, M V Soloviev, F David, S Desrochers, H Brunengraber
Index: Anal. Biochem. 221(2) , 323-8, (1994)
Full Text: HTML
Abstract
We developed gas chromatographic-mass spectrometric assays for the enantiomers of 1,2-propanediol, 1,3-butanediol, 1,3-pentanediol, and their corresponding hydroxyacids, lactate, beta-hydroxybutyrate, and beta-hydroxypentanoate (3-hydroxyvalerate) in biological fluids. The corresponding ketoacids, acetoacetate and beta-ketopentanoate, can be assayed simultaneously by pretreating the samples with NaB2H4. The assays involve spiking the samples with deuterated internal standards, deproteinization, ether extraction, and derivatization of the carboxyl groups with (R,S)-2-butanol/HCl and of the hydroxyl groups with chiral (S)-(+)-2-phenylbutyryl chloride. Mass spectrometric analysis is conducted under ammonia positive chemical ionization. We used these assays to follow the metabolism of diol enantiomers in dogs. For (R,S)-1,3-butanediol and (R,S)-1,3-pentanediol, the uptakes from dog plasma of the R and S enantiomer of each diol were identical. In contrast, the metabolism of (S)-1,2-propanediol was faster than that of (R)-1,2-propanediol. (R)-1,2-Propanediol is formed during acetone metabolism, while (R,S)-1,3-butanediol and (R,S)-1,3-pentanediol are potential nutrients. The assays developed will allow further investigations of the metabolisms of acetone, (R)-lactate, and artificial nutrients derived from the 1,3-butanediol and 1,3-pentanediol enantiomers.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
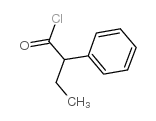 |
2-Phenylbutyryl chloride
CAS:36854-57-6 |
C10H11ClO |
|
Determination of dopamine and dopamine-derived (R)-/(S)-sals...
2000-09-11 [Forensic Sci. Int. 113(1-3) , 359-66, (2000)] |
|
Determining the absolute configuration of hindered secondary...
[Tetrahedron Lett. 30(28) , 3629-32, (1989)] |
|
Novel synthesis of. alpha.,. beta.-unsaturated ketones by th...
[J. Org. Chem. 52(15) , 3186-92, (1987)] |