Supramolecular architectures and structural diversity in a series of lead (II) Chelates involving 5-Chloro/Bromo thiophene-2-carboxylate and N,N'-donor ligands.
Samson Jegan Jennifer, Packianathan Thomas Muthiah
Index: Chem. Cent. J. 7 , 139, (2013)
Full Text: HTML
Abstract
Lead is a heavy toxic metal element in biological systems and is one of the major pollutants as a result of its widespread use in industries. In spite of its negative roles the coordination chemistry of Pb(II) complexes is a matter of interest. The N,N'-bidentate aromatic bases such as BPY,4-BPY and PHEN (BPY = 2,2'bipyridine, 4-BPY = 4,4'-dimethyl-2,2'-bipyridine, PHEN = 1,10-Phenanthroline) are widely used to build supramolecular architectures because of their excellent coordinating ability and large conjugated system that can easily form π-π interactions among their aromatic moieties. A series of novel Pb(II) complexes in concert with 5-CTPC, 5-BTPC (5-CTPC = 5-chlorothiophen-2-carboxylate, 5-BTPC = 5-bromothiophen-2-carboxylate) and corresponding bidentate chelating N.N' ligands have been synthesized and characterized.Five new Pb (II) complexes [Pb(BPY)(5-CTPC)2] (1), [Pb(4-BPY)(5-CTPC)2] (2), [Pb2(PHEN)2(5-CTPC)4] (3), [Pb(4-BPY)(5-BTPC)2] (4) and [Pb2(PHEN)2(5-BTPC)2(ACE)2] (5) have been synthesized. Even though in all these complexes the molar ratio of Pb, carboxylate, N,N-chelating ligand are the same (1:2:1), there is a significant structural diversity. These complexes have been characterised and investigated by elemental analysis, IR, 1H-NMR,13C-NMR, TGA, and photoluminescence studies. Single crystal X-ray diffraction studies reveal that complexes (1, 2) and (4) are mononuclear while (3 and 5) are dinuclear in nature which may result from the chelating nature of the ligands, various coordination modes of the carboxylates, and the coordination geometry of the Pb(II) ions.The observation of structures 2,4 and 3,5 show the structural changes made just chloro/bromo substituent of the thiophene ring. A detailed packing analysis has been undertaken to delineate the role of valuable non covalent interactions like X…π, H…X, (X = Cl/Br). A quadruple hydrogen bond linking the monomeric units and generating a supramolecular architecture is observed in (1). The metal bite unit comprised of PbN2C2 (i.e. Pb-N-C-C-N-Pb) is the repeating unit in all the five complexes and they have almost same geometrical parameters. This metal bite has been identified as the self assembly unit in complexes.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
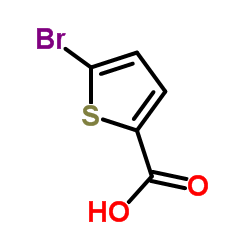 |
5-Bromo-2-thiophenecarboxylic acid
CAS:7311-63-9 |
C5H3BrO2S |
|
Synthesis and characterization of donor-π-acceptor-based por...
2014-10-20 [Chemistry 20(43) , 14074-83, (2014)] |
|
Oligothiophenes as fluorescent markers for biological applic...
2012-01-01 [Molecules 17(1) , 910-33, (2012)] |
|
Effect of ester moieties in dye structures on photoinduced r...
[Chem. Mater. 17(17) , 4304-4309, (2005)] |
|
THE SYNTHESIS AND CHARACTERIZATION OF AMPHIPHILIC a, a'-DISU...
[Polymer Prepr. 47(20 , 787, (2006)] |