Further studies on the spectrophotometric determination of amprolium.
David W Fink, Gerald deFontenay, Philippe Bonnefille, Monique Camarade, Charles Monier
Index: J. AOAC Int. 87(3) , 677-80, (2004)
Full Text: HTML
Abstract
The official AOAC spectrophotometric analytical method for amprolium in feeds (961.24) is quantitatively selective for the intact drug in the presence of its primary degradation products. Concentrations evaluated included mixtures of the individual degradates in the presence of amprolium, as well as an equimolar mixture of the 2 degradates. Neither compound responds to the amprolium colorimetric derivatization reaction under any conditions, demonstrating that the official method can be used as an analytical technique for demonstrating the stability of amprolium in medicated feeds. Additionally, liquid chromatography conditions have been established to resolve amprolium from its degradation products.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
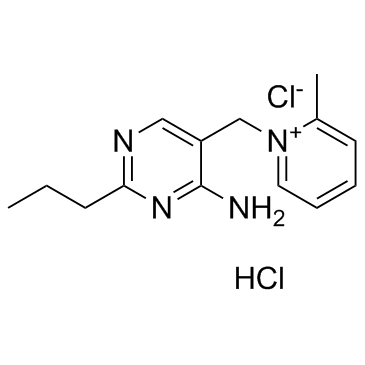 |
Amprolium Hydrochloride
CAS:137-88-2 |
C14H20Cl2N4 |
|
Veterinary drug residues in domestic and imported foods of a...
2015-01-01 [Food. Addit. Contam. Part. B. Surveill. 8 , 106-12, (2015)] |
|
Ethanol promotes thiamine deficiency-induced neuronal death:...
2009-06-01 [Alcohol. Clin. Exp. Res. 33(6) , 1097-103, (2009)] |
|
Determination of amprolium in feed by a liquid chromatograph...
2008-12-15 [J. Pharm. Biomed. Anal. 48(5) , 1457-61, (2008)] |
|
Three thiamine analogues differently alter thiamine transpor...
2003-12-01 [Metab. Brain Dis. 18(4) , 245-63, (2003)] |
|
Acetyl-CoA metabolism in amprolium-evoked thiamine pyrophosp...
2011-08-01 [Neurochem. Int. 59(2) , 208-16, (2011)] |