| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ammonium hexachloroiridate(IV)
CAS:16940-92-4 |
|
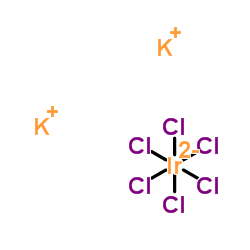 |
Dipotassium hexachloroiridate(2-)
CAS:16920-56-2 |