Asymmetric synthesis of novel thioiso dideoxynucleosides with exocyclic methylene as potential antiviral agents.
Prashantha Gunaga, Masanori Baba, Lak Shin Jeong
Index: J. Org. Chem. 69(9) , 3208-11, (2004)
Full Text: HTML
Abstract
Novel thioiso pyrimidine and purine nucleosides substituted with exocyclic methylene have been synthesized, starting from D-xylose. The glycosyl donor 14 was synthesized from D-xylose, using cyclization of dimesylate 10 with sodium sulfide as a key step. Cyclization proceeded in pure S(N)2 reaction without going through S(N)1 reaction in the presence of an allylic functional group at low reaction temperature (0 degrees C) in polar solvent (DMF), affording compound 12 as a major product. At higher temperatures, S(N)2' product 11 was almost exclusively obtained as a major product. On the other hand, glycosylation of 14 with 6-chloropurine under Mitsunobu conditions afforded the desired S(N)2 product 26, while palladium-catalyzed glycosylation resulted in the sole formation of S(N)2' product 34.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
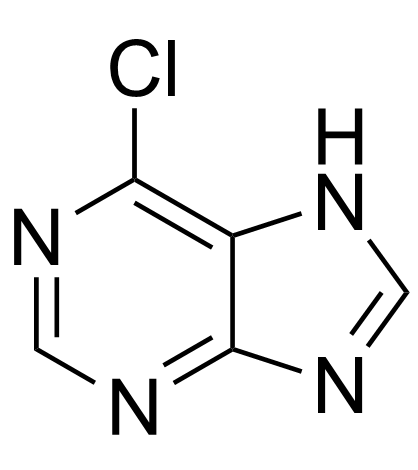 |
6-chloropurine
CAS:87-42-3 |
C5H3ClN4 |
|
Synthesis of modified homo-N-nucleosides from the reactions ...
2009-01-01 [Bioorg. Med. Chem. Lett. 19 , 6433-6, (2009)] |
|
The synthesis of novel fluorescent purine analogues modified...
2010-01-01 [Bioorg. Med. Chem. Lett. 20(10) , 3098-102, (2010)] |
|
Evidence for Watson-Crick and not Hoogsteen or wobble base p...
2005-03-29 [Biochemistry 44(12) , 4850-60, (2005)] |
|
Synthesis and biological evaluation of nucleoside analogues ...
2007-05-01 [Bioorg. Med. Chem. Lett. 17 , 2470-3, (2007)] |
|
Microwave-assisted amination of a chloropurine derivative in...
2005-01-01 [Molecules 10(2) , 508-15, (2005)] |