Formation of the Vilsmeier-Haack complex: the performance of different levels of theory.
Gül Altınbaş Ozpınar, Dieter E Kaufmann, Timothy Clark
Index: J. Mol. Model. 17(12) , 3209-17, (2011)
Full Text: HTML
Abstract
Because of discrepancies in the available experimental data, an extensive theoretical investigation of the formation of the Vilsmeier-Haack (VH) complex has been carried out. The barriers to complex formation calculated using eight different density functional methods (BLYP, B2-PLYP, B3LYP, B3PW91, MPW1K, M06-2X, and PBE1PBE), MP2, and extrapolation techniques (CBS-QB3, G3B3) with several basis sets (6 - 31 + G, 6 - 311++G, 6 - 311 + (3df,2p), aug-cc-pVDZ, and aug-cc-pVTZ) were compared with experimental data. For the overall reaction, MP2/aug-cc-pVDZ and M06-2X/6-31 + G(d,p) perform best compared to the CBS techniques. The results help clarify some open mechanistic questions.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
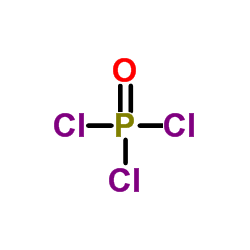 |
phosphoryl trichloride
CAS:10025-87-3 |
Cl3OP |
|
Design of donor-acceptor star-shaped oligomers for efficient...
2014-01-01 [Faraday Discuss. 174 , 313-39, (2014)] |
|
Toughened hydrogels inspired by aquatic caddisworm silk.
2015-09-21 [Soft Matter 11 , 6981-90, (2015)] |
|
Differentiation between acetylcholinesterase and the organop...
2003-11-14 [J. Biol. Chem. 278(46) , 45512-8, (2003)] |
|
POCl3 chlorination of 4-quinazolones.
2011-03-18 [J. Org. Chem. 76(6) , 1653-61, (2011)] |
|
Facile small scale synthesis of nucleoside 5'-phosphate mixt...
2010-01-01 [Nucleosides Nucleotides Nucleic Acids 29(1) , 14-26, (2010)] |