From symmetric glycerol derivatives to dissymmetric chlorohydrins.
Carmen Solarte, Marc Escribà, Jordi Eras, Gemma Villorbina, Ramon Canela, Mercè Balcells
Index: Molecules 16(3) , 2065-74, (2011)
Full Text: HTML
Abstract
The anticipated worldwide increase in biodiesel production will result in an accumulation of glycerol for which there are insufficient conventional uses. The surplus of this by-product has increased rapidly during the last decade, prompting a search for new glycerol applications. We describe here the synthesis of dissymmetric chlorohydrin esters from symmetric 1,3-dichloro-2-propyl esters obtained from glycerol. We studied the influence of two solvents: 1,4-dioxane and 1-butanol and two bases: sodium carbonate and 1-butylimidazole, on the synthesis of dissymmetric chlorohydrin esters. In addition, we studied the influence of other bases (potassium and lithium carbonates) in the reaction using 1,4-dioxane as the solvent. The highest yield was obtained using 1,4-dioxane and sodium carbonate.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
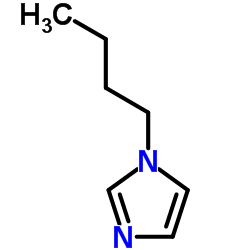 |
1-butylimidazole
CAS:4316-42-1 |
C7H12N2 |
|
Synthesis of cationic single-isomer cyclodextrins for the ch...
2007-01-01 [Nat. Protoc. 2(12) , 3195-200, (2007)] |
|
Simple chip-based interfaces for on-line monitoring of supra...
2005-10-01 [Lab Chip 5(10) , 1111-22, (2005)] |
|
Anhydrous proton transport system based on zwitterionic liqu...
2004-08-21 [Chem. Commun. (Camb.) 16 , 1828-29, (2004)] |
|
Synthesis of mesomeric betaine compounds with imidazolium-en...
2012-01-01 [Beilstein J. Org. Chem. 8 , 390-7, (2012)] |
|
Homoleptic and heteroleptic N-alkylimidazole zinc(ii)-contai...
2014-08-28 [Dalton Trans. 43(32) , 12329-41, (2014)] |