| Structure | Name/CAS No. | Articles |
|---|---|---|
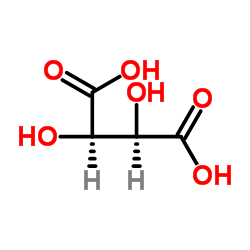 |
DL-Tartaric acid
CAS:133-37-9 |
|
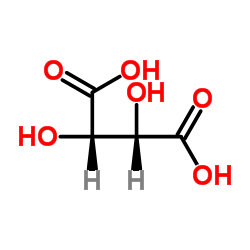 |
Tartaric acid
CAS:87-69-4 |
|
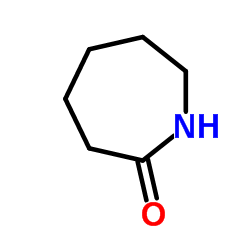 |
Caprolactam
CAS:105-60-2 |
|
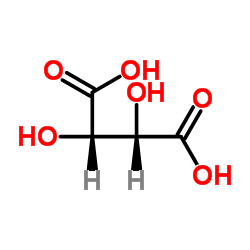 |
D-Tartaric acid
CAS:147-71-7 |