| Structure | Name/CAS No. | Articles |
|---|---|---|
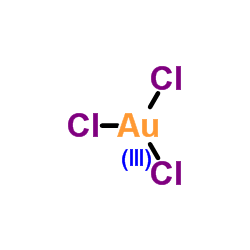 |
Gold(III) chloride
CAS:13453-07-1 |
|
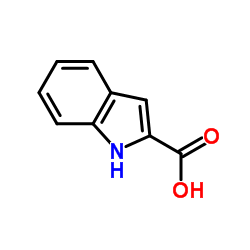 |
Indole-2-carboxylic acid
CAS:1477-50-5 |
|
 |
Gold (I) chloride
CAS:10294-29-8 |