Convenient synthesis of polyfunctional dihydrothiophenes with tandem reaction of 1,3-thiazolidinedione, aldehyde, arylamine and ethyl cyanoacetate.
Jing Sun, Er-Yan Xia, Rong Yao, Chao-Guo Yan
Index: Mol. Divers. 15(1) , 115-23, (2011)
Full Text: HTML
Abstract
New, highly-functionalized dihydrothiophenes are conveniently synthesized from the novel tandem, four-component reactions of 1,3-thiazolidinedione, aldehyde, arylamine, and ethyl cyanoacetate, catalyzed by triethylamine. The reaction mechanism involves the use of Knoevenagel condensation, Michael addition, and ring-opening of 1,3-thiazolidinedione, followed by intramolecular ring closure process. The reaction is diastereoselective and only trans-2,3-dihydrothiophenes were produced in moderate yields. The functionalized dihydrothiophenes can be converted efficiently to the corresponding thiophenes by DCC dehydrogenation reaction.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
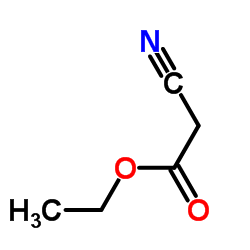 |
Ethyl cyanoacetate
CAS:105-56-6 |
C5H7NO2 |
|
Self-assembled hybrid metal oxide base catalysts prepared by...
2015-01-01 [Nat. Commun. 6 , 8580, (2015)] |
|
Intramolecular Hydrogen Bonding and Linear Pentapyrrole Conf...
2014-08-01 [Monatsh. Chem 145(7) , 1117-1135, (2014)] |
|
Microwave-assisted and efficient solvent-free knoevenagel co...
2010-02-01 [Molecules 15(2) , 813-23, (2010)] |
|
Synthesis of indan-based unusual alpha-amino acid derivative...
2000-03-10 [J. Org. Chem. 65(5) , 1359-65, (2000)] |
|
Direct organocatalytic and highly enantio- and diastereosele...
2005-05-06 [Angew. Chem. Int. Ed. Engl. 44(19) , 2896-9, (2005)] |