| Structure | Name/CAS No. | Articles |
|---|---|---|
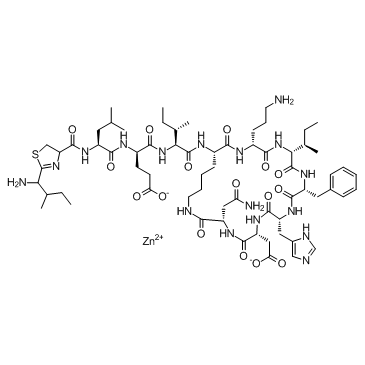 |
Bacitracin zinc
CAS:1405-89-6 |
|
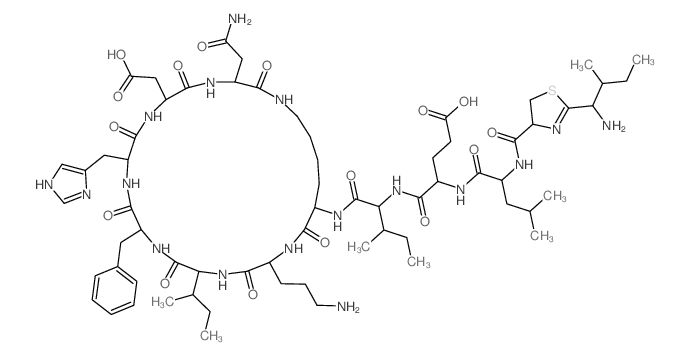 |
Bacitracin A1 (9CI)
CAS:22601-59-8 |
|
 |
Bacitracin
CAS:1405-87-4 |