| Structure | Name/CAS No. | Articles |
|---|---|---|
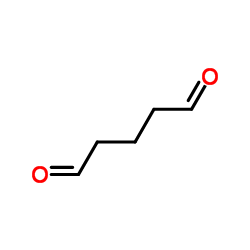 |
glutaraldehyde
CAS:111-30-8 |
|
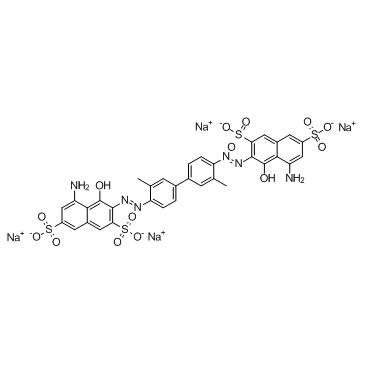 |
Direct Blue 14
CAS:72-57-1 |
|
 |
2-(Hydroxymethyl)-2-nitropropan-1,3-diol
CAS:126-11-4 |
|
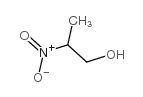 |
2-nitropropan-1-ol
CAS:2902-96-7 |
|
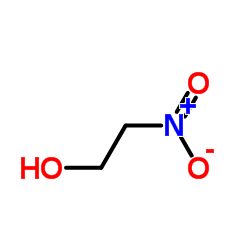 |
2-Nitroethanol
CAS:625-48-9 |