Deglycosylation systematically improves N-glycoprotein identification in liquid chromatography-tandem mass spectrometry proteomics for analysis of cell wall stress responses in Saccharomyces cerevisiae lacking Alg3p.
Ulla-Maja Bailey, Benjamin L Schulz
Index: J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 923-924 , 16-21, (2013)
Full Text: HTML
Abstract
Post-translational modification of proteins with glycosylation is of key importance in many biological systems in eukaryotes, influencing fundamental biological processes and regulating protein function. Changes in glycosylation are therefore of interest in understanding these processes and are also useful as clinical biomarkers of disease. The presence of glycosylation can also inhibit protease digestion and lower the quality and confidence of protein identification by mass spectrometry. While deglycosylation can improve the efficiency of subsequent protease digest and increase protein coverage, this step is often excluded from proteomic workflows. Here, we performed a systematic analysis that showed that deglycosylation with peptide-N-glycosidase F (PNGase F) prior to protease digestion with AspN or trypsin improved the quality of identification of the yeast cell wall proteome. The improvement in the confidence of identification of glycoproteins following PNGase F deglycosylation correlated with a higher density of glycosylation sites. Optimal identification across the proteome was achieved with PNGase F deglycosylation and complementary proteolysis with either AspN or trypsin. We used this combination of deglycosylation and complementary protease digest to identify changes in the yeast cell wall proteome caused by lack of the Alg3p protein, a key component of the biosynthetic pathway of protein N-glycosylation. The cell wall of yeast lacking Alg3p showed specifically increased levels of Cis3p, a protein important for cell wall integrity. Our results showed that deglycosylation prior to protease digestion improved the quality of proteomic analyses even if protein glycosylation is not of direct relevance to the study at hand.Copyright © 2013 Elsevier B.V. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
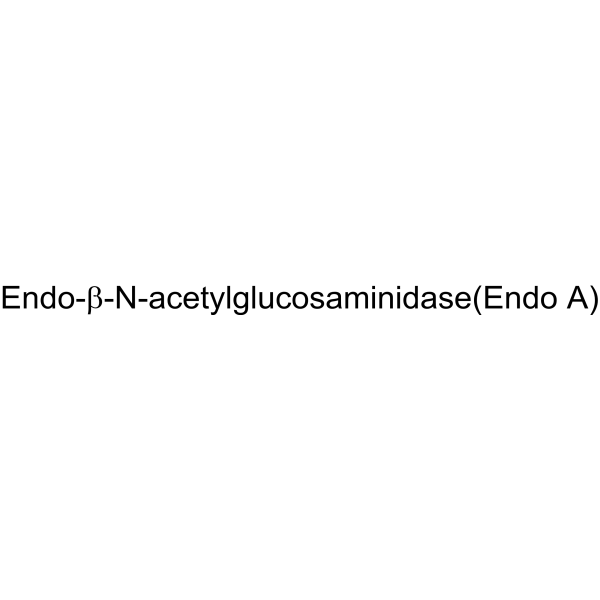 |
Endo-β-N-acetylglucosaminidase (Endo A)
CAS:37278-88-9 |
|
Improved plasma membrane expression of the trafficking defec...
2015-03-01 [Int. J. Biochem. Cell Biol. 60 , 119-29, (2015)] |
|
A cholesterol consensus motif is required for efficient intr...
2015-01-15 [Biochem. J. 465(2) , 305-14, (2015)] |
|
Proteome analysis of the farnesol-induced stress response in...
2012-07-16 [J. Proteomics 75(13) , 4038-49, (2012)] |
|
The hepatitis C virus E1 glycoprotein undergoes productive f...
2011-01-01 [PLoS ONE 6(8) , e23838, (2011)] |
|
An endo-β-N-acetylglucosaminidase from Enterococcus faecalis...
2011-12-01 [FEMS Microbiol. Lett. 325(2) , 123-9, (2011)] |