Metabolism of Halophenols by 2,4,5-trichlorophenoxyacetic acid-degrading Pseudomonas cepacia.
J S Karns, J J Kilbane, S Duttagupta, A M Chakrabarty
Index: Appl. Environ. Microbiol. 46(5) , 1176-81, (1983)
Full Text: HTML
Abstract
Resting cells of 2,4,5-trichlorophenoxyacetic acid-grown Pseudomonas cepacia AC1100 were able to completely and rapidly dechlorinate several chlorine-substituted phenols, including 2,4,5-trichlorophenol, 2,3,4,6-tetrachlorophenol, and pentachlorophenol. Several other trichlorophenols were only partially dechlorinated. The evidence suggests that 2,4,5-trichlorophenol is an intermediate in the degradation of 2,4,5-trichlorophenoxyacetic acid by strain AC1100. Moreover, although strain AC1100 was isolated by selection for growth on a chlorinated aromatic compound, brominated and fluorinated analogs were efficiently dehalogenated by strain AC1100 resting cells, whereas an iodinated analog was poorly dehalogenated.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
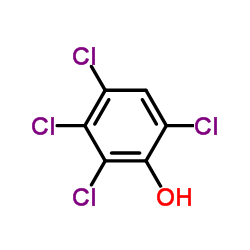 |
2,3,4,6-TETRACHLOROPHENOL
CAS:58-90-2 |
C6H2Cl4O |
|
PCDD/F formation from chlorophenols by lignin and manganese ...
2014-09-01 [Chemosphere 110 , 129-35, (2014)] |
|
Comparison of stir bar sorptive extraction and solid-phase m...
2008-05-15 [Talanta 75(3) , 753-9, (2008)] |
|
In situ polychlorophenol bioremediation potential of the ind...
2001-07-01 [Water Res. 35(10) , 2496-504, (2001)] |
|
Chemical kinetic modelling of PCDD formation from chlorophen...
2000-09-01 [Chemosphere 41(6) , 943-51, (2000)] |
|
Enhanced solubilization of arsenic and 2,3,4,6 tetrachloroph...
2004-10-01 [Chemosphere 57(3) , 197-206, (2004)] |