A chemical synthesis of 11-methoxy mitragynine pseudoindoxyl featuring the interrupted Ugi reaction.
Jimin Kim, JohnS Schneekloth, ErikJ Sorensen
Index: Chem. Sci. 3(9) , 2849-2852, (2012)
Full Text: HTML
Abstract
A synthesis of 11-methoxy mitragynine pseudoindoxyl, a new member of the mitragynine class of opioid agonists, from a derivative of the Geissman-Waiss lactone is described. An internal attack of an electron-rich aromatic ring on an electrophilic nitrilium ion and a late-stage construction of the functionalized piperidine ring by the method of reductive cyclization are the pivotal transformations; both ring annulations proceed in a highly diastereoselective fashion. The construction of substituted indoxyl frameworks by the interrupted Ugi method offers an attractive alternative to the strategy of oxidatively rearranging indoles.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
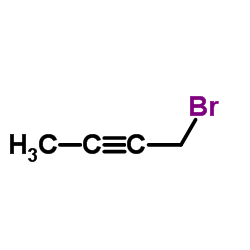 |
1-Bromo-2-butyne
CAS:3355-28-0 |
C4H5Br |
|
Iridium complex-catalyzed highly enantio- and diastereoselec...
2004-07-14 [J. Am. Chem. Soc. 126(27) , 8382-8383, (2004)] |
|
Imaging breakdown diagrams for bromobutyne isomers with phot...
[Phys. Chem. Chem. Phys. 16(2) , 505-515, (2014)] |
|
Facile Entry to 4, 5, 6, 7-Tetrahydro [1, 2, 3] triazolo [1,...
[European J. Org. Chem. 2008(14) , 2423-2429, (2008)] |
|
Acetylenic TACE inhibitors. Part 1. SAR of the acyclic sulfo...
[Bioorg. Med. Chem. Lett. 13(16) , 2799-2803, (2003)] |
|
Reaction of zirconacyclopentadienes with electrophiles such ...
[Tetrahedron Lett. 45(49) , 9041-9043, (2004)] |