Specific cleavage of amino side chains of serine and threonine in peptides and proteins with S-ethyltrifluorothioacetate vapor.
M Kamo, A Tsugita
Index: Eur. J. Biochem. 255(1) , 162-71, (1998)
Full Text: HTML
Abstract
A vapor of S-ethyltrifluorothioacetate was found to specifically cleave the amino side of serine and threonine peptide bonds. The cleavage reactions were carried out at 50 degrees C for 6 h-24 h or at 30 degrees C for 24 h. When vapors were generated in a solution containing several conventional organic solvents, the cleavage reactions were reduced or stopped, or modification took place. When the reagent vapor was made in an aqueous solution, the cleavage reaction at glycine residues was enhanced. This reagent did not oxidize any amino acid residues, such as methionine, cysteine and tryptophan. The cleavage was also effective on proteins on membranes blotted or electroblotted from polyacrylamide gels. This method therefore may be used for the peptide mass fingerprinting [Patterson, S. D. (1995) Electrophoresis 16, 1104-1114] after two-dimensional electrophoresis.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
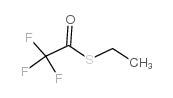 |
Trifluoroacetonylmercaptoethanol
CAS:383-64-2 |
C4H5F3OS |
|
Fluorinated proteins as potential 19F magnetic resonance ima...
1994-01-01 [Bioconjug. Chem. 5(3) , 257-61, (1994)] |
|
The primary structure of mitochondrial aspartate aminotransf...
1979-01-01 [Ital. J. Biochem. 28(6) , 441-55, (1979)] |
|
Investigation of protein structure by means of 19F-NMR. A st...
1988-11-01 [Eur. J. Biochem. 177(2) , 383-94, (1988)] |
|
Multiple-sites C-terminal sequencing methods of protein and ...
1998-08-01 [J. Protein Chem. 17(6) , 520-1, (1998)] |
|
A conformational and vibrational study of CF(3)COSCH(2)CH(3)...
2009-12-07 [J. Chem. Phys. 131(21) , 214303, (2009)] |