A practical preparation of the key intermediate for penems and carbapenems synthesis.
Barbara Grzeszczyk, Sebastian Stecko, Łukasz Mucha, Olga Staszewska-Krajewska, Marek Chmielewski, Bartłomiej Furman
Index: J. Antibiot. 66(3) , 161-3, (2013)
Full Text: HTML
Abstract
A novel, practical and stereoselective synthesis of (3R,4R)-4-acetoxy-3-[(R)-1-(t-butyldimethylsilyloxy)ethyl]-2-azetidinone, a key intermediate in the preparation of β-lactam antibiotics is reported. The crucial step of the synthesis is based on the Cu(I)-mediated Kinugasa cycloaddition/rearrangement cascade between silyl protected (R)-3-butyn-2-ol and the nitrone derived from benzyl hydroxylamine and benzyl glyoxylate. The obtained adduct is subjected to debenzylation with sodium, or lithium in liquid ammonia followed by oxidation with lead tetraacetate to afford the final product.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
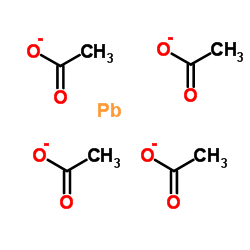 |
lead tetraacetate
CAS:546-67-8 |
C8H12O8Pb |
|
Chlorodecarboxylation of 17 beta-acetoxy-3-methoxy-9-oxo-9, ...
1982-05-01 [Steroids 39(5) , 537-45, (1982)] |
|
Formation of an unusual dimeric compound by lead tetraacetat...
2002-07-01 [Chem. Pharm. Bull. 50(7) , 960-3, (2002)] |
|
An efficient synthesis of 4 beta- and 6 alpha-hydroxylated b...
1993-02-01 [Steroids 58(2) , 52-8, (1993)] |
|
Synthesis of c-2, 3, 17 and 19-oxygenated androgens.
1988-03-01 [J. Steroid Biochem. 29(3) , 353-9, (1988)] |
|
First chemical synthesis of antioxidative metabolites of ses...
2008-11-01 [Chem. Pharm. Bull. 56(11) , 1611-2, (2008)] |