| Structure | Name/CAS No. | Articles |
|---|---|---|
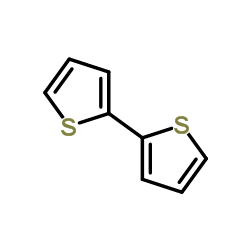 |
2,2'-Bithiophene
CAS:492-97-7 |
|
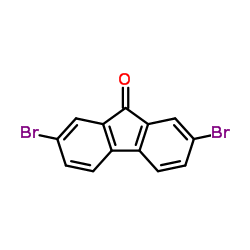 |
2,7-dibromo-9-fluorenone
CAS:14348-75-5 |
|
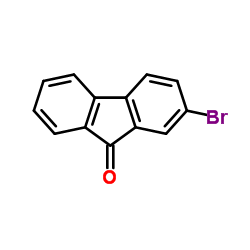 |
2-bromo-9-fluorenone
CAS:3096-56-8 |