| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
(S)-(-)-1,2-Diaminopropane dihydrochloride
CAS:19777-66-3 |
|
 |
(2R)-1,2-Propanediamine dihydrochloride
CAS:19777-67-4 |
|
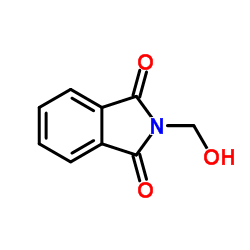 |
2-(Hydroxymethyl)isoindoline-1,3-dione
CAS:118-29-6 |
|
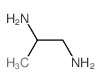 |
1,2-Diaminopropane
CAS:78-90-0 |